NIH clinical trial shows silver diamine fluoride more effective than placebo in treating pediatric cavities
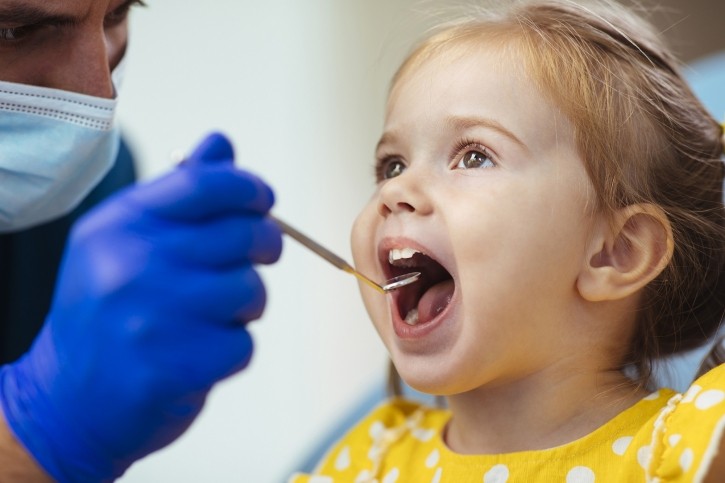
The preliminary results of a recent National Institute of Dental and Craniofacial Research (NIDCR) funded study published in Pediatric Dentistry have determined that silver diamine fluoride (SDF), a topical liquid, can put an end to tooth decay in pediatric patients. As reported in a National Institute of Health press release, the treatment stopped the progression of 54% of cavities “compared to 21% of those treated with a placebo.”
As detailed in the NIH press release, “SDF is cleared by the Food and Drug Administration for treating dental sensitivity and is used off label to treat tooth decay” through non-invasive topical application. Having “been widely used for management of tooth decay in other countries for decades,” the release continued, previous “studies suggest that the silver in SDF kills cavity-causing microbes and helps stop destruction of the tooth, while the fluoride helps to rebuild and strengthen the tooth.”
About the study
As detailed in the study overview, the trial, which began in October of 2018 and will be completed next month, “is a phase III, multicenter, randomized, placebo-controlled superiority trial, with two parallel groups (placebo vs. SDF applied to cavitated lesions) involving a total of 1144 children with the primary outcome assessed at six months after initial treatment.”
Child participants ranged between one and five years of age and were recruited from “early childhood education programs, such as Head Start centers, Early Head Start centers, and equivalent city/state subsidized preschool programs, or children attending dental clinics associated with the clinical trial sites” who demonstrated signs of severe tooth decay, the study overview explained.
During the study, visits occurred “at baseline, three months, six months, and eight months [with] SDF/placebo…applied at baseline and six months” accompanied by intermediate contacts with researchers to “determine if the child needs an additional visit to assess pain, lesion progression, etc. and to maintain contact with the participant,” the overview continued.
To determine the efficacy of the treatment, “at each clinical visit International Caries Detection and Assessment System (ICDAS) examinations, including cavitated lesion hardness assessments, soft tissue assessments and questionnaires on dental discomfort, family impact and treatment satisfaction and acceptability” were recorded.
Study determinations
“An interim analysis of 599 children evaluated the proportion of cavity lesions in which decay progression was stopped six months after a single treatment with SDF or a placebo,” the NIH press release stated, and “the team reported no safety concerns with the treatment.”
Additionally, “because the planned interim analysis revealed that the study had met its primary endpoint — to show that SDF was effective in halting the progression of cavities — the trial was stopped early,” the release continued, because “ending a trial early can allow particularly effective interventions to move toward FDA approval, and thus, availability to patients, sooner.”
Currently, “researchers are now analyzing the final data on over 800 children, including assessing SDF’s effects on tooth pain and quality of life, as well as potential side effects,” the release said. One potentially unwanted side effect of the SDF treatment is that the treated cavity area can darken in color, “which may not be aesthetically pleasing, [so] the researchers are also evaluating patient and parent satisfaction and acceptability.”
Potential impact of study results
The scope of the study and its results are significant as “tooth decay is the most common chronic disease of childhood, affecting nearly 46% of children in the United States, according to the Centers for Disease Control and Prevention,” the NIH press release noted. “Left untreated, cavities can put children at risk for chronic pain, impaired development, and long-term oral and overall health problems,” and “in severe cases, bacteria from a tooth infection can even travel through the body and cause death,” added the release.
Additionally, “current treatments for severe early childhood caries rely on restoration and tooth extraction, which can involve general anesthesia,” reported lead investigator Margherita Fontana, D.D.S., Ph.D., of the University of Michigan in the NIH release. “This study provides evidence that SDF could be a powerful tool against cavities and help improve health and well-being of children,” added NIDCR Director Rena D’Souza, D.D.S., Ph.D., M.S.