Analytical laboratory Valisure submits Citizen’s Application to FDA requesting ‘a recall and suspension of sale of products containing benzoyl peroxide’
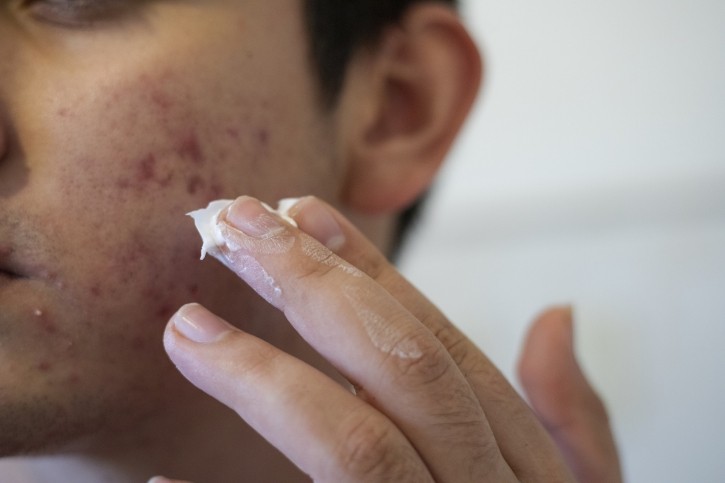
Last week, independent laboratory Valisure submitted a formal Citizen’s Application to the FDA “to request the Commissioner of Food and Drugs to issue a regulation, revise industry guidance, and request a recall and suspend sales of benzoyl peroxide from the US market” following the results of recent testing which revealed “that benzene, a known human carcinogen, can form at high levels in Benzoyl Peroxide ("BPO") acne treatment products,” the company confirmed.
In a company press release, Valisure asserted that the laboratory’s testing “on dozens of prescriptions and over-the-counter benzoyl peroxide products suggest that currently formulated BPO medications are fundamentally unstable and can generate unacceptably high levels of benzene when handled or stored at higher temperatures that the products may be exposed to during handling by consumers.”
The company’s Citizen’s Application to the FDA, which was submitted on March 5 of this year, outlines the historical research into benzene’s classification as a known carcinogen, a summary of Valisure’s testing and results analysis, and the actions to be undertaken by the FDA to address the risk of potential benzene exposure to consumers via benzoyl peroxide acne products.
Benzene and BPO
As noted in Valisure’s Citizen’s Application to the FDA, “benzene has long been directly associated with cancer in humans by epidemiological studies with persistent exposure as low as 0.8 ppm.”
Historical research into benzene’s carcinogenic properties dates back over a century, the application further said, citing “a study from 1939 on benzene [which] stated that ‘exposure over a long period of time to any concentration of benzene greater than zero is not safe,’ which is a comment reiterated in a 2010 review of benzene research specifically stating, ‘There is probably no safe level of exposure to benzene, and all exposures constitute some risk in a linear if not supralinear, and additive fashion.’”
The application also cites information from the American Cancer Society, which explains that the risks of benzene exposure include “sufficient evidence that benzene causes acute myeloid leukemia (AML)…benzene exposure has been linked with acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), multiple myeloma, and non-Hodgkin lymphoma.”
Regarding the FDA’s current regulatory actions regarding benzene in consumer product formulations, the application notes that “FDA currently recognizes the danger of benzene and, as a result, has claimed it should not be used in the manufacture of any component of a drug product, and only if its use is “unavoidable” should a strict concentration limit of 2ppm apply,” Valisure stated.
In its application, Valisure emphasizes that “it is important to note that the specific problem with benzene in benzoyl peroxide products does not appear to be a contamination issue from a specific ingredient, but instead the inherent instability of the benzoyl peroxide molecule that breaks down and forms benzene,” which is a key element of the organization’s argument for action from the FDA regarding the issue with BPO-containing acne products.
Further, Valisure stated in its application that the organization was “not aware of any FDA guidance on a total amount, or permissible daily exposure limit, for benzene in any drug or cosmetic product, including benzoyl peroxide products, and requests urgent action on behalf of FDA to issue guidance to fill this gap,” and noted precedence by the FDA on addressing the issue of benzene contamination in previous products, including hand sanitizers, sun and body care products, and dry shampoos.
Testing results in support of Valisure’s Citizen’s Application
As detailed in its application, Valisure’s initial analysis was performed on 175 acne treatment products and of those “99 products contained BPO as at least one of the active ingredients, 58 products contained active ingredients either individually or in combination with salicylic acid (46 of 58 products), sulfur, adapalene, azelaic acid, niacinamide and zinc, and 18 products contained no drug ingredient,” with 83 of the BPO products being OTC and 16 being prescriptions purchased from a licensed wholesaler.
Of important note, “analysis for native benzene was performed on 99 BPO containing acne medication products and resulted in the detection of benzene in 94 products, often with values well above 2ppm in an initial analysis.”
As a result, “this high prevalence of benzene in specifically BPO acne treatment products and discovering research in the academic literature as early as 1936 that concluded BPO can directly degrade into benzene led Valisure to conduct a stability study at elevated temperatures on a diverse market sweep of BPO products and formulations….[and] 50°C (122°F) was chosen as a stability temperature for a broader study of 66 BPO products.”
The application goes on to report that “results from this 50°C stability study showed substantial instability of BPO and its propensity to form concerningly high levels of benzene in only 18 days,” which is a crucial finding as “Petitioner notes that 50°C (122°F) is not only a reasonable temperature that ‘the product may be exposed to during distribution and handling by consumers’ but is an accepted incubation temperature used by the pharmaceutical industry for performing accelerated stability studies with a duration of at least three months.”
Additionally, during its testing, “Valisure observed evidence that some BPO-containing acne treatment products may have packaging that is porous to benzene, enabling some portion of benzene contained in the product, or formed in the product during stability testing, to escape into the environment or air around the package,” the application stated.
Further testing was therefore conducted “to evaluate levels of benzene in the air surrounding an incubating BPO product,” and the results suggest that “high levels of gaseous benzene could be generated from a BPO-containing product and emanate into a consumer environment such as a hot car or bathroom during a hot shower,” confirming that “benzene can leak outside of unopened BPO containing acne treatment products at concerningly high levels.”
Requested actions to address benzene contamination in BPO acne products
In its application, Valisure requested a set of nine actions or interventions by the FDA to address the “serious concern over the safety of drug products formulated with BPO,” specifically the laboratory’s “current evidence, [which] suggests that on-market BPO products could produce substantial amounts of benzene when stored at above-ambient temperatures, specifically 37°C (98.6°F), 50°C (122°F) and 70°C (158°F).”
Among the requested actions to address benzene contamination in BPO acne products is that the Citizen’s Application “seeks to have the Commissioner and FDA request recalls and a suspension of sales for products containing the active pharmaceutical ingredient benzoyl peroxide, consistent with FDA’s mandate to ensure the safety of prescription and over the counter the drugs in the United States.”
The application goes on to note that “such actions are extremely important for public safety,” and that “the elimination of benzoyl peroxide from the market is not expected to create a significant impact on the U.S. healthcare system or patients currently using the drug due to the fact that many alternatives exist for the treatment of acne vulgaris,” as “Valisure specifically tested other drug products, like salicylic acid, and did not find any evidence of benzene formation.”
Other requested actions include “that the FDA promulgate regulations requiring robust independent chemical testing and verification of medications,” and “in the interim, while these regulations are pending, FDA should issue formal guidance recommending such testing and verification,” stated the application.